Contents Catalog
Introduction of Temperature on Lime Slaking Process
Lime slaking is critical in various industrial applications, particularly in water treatment, paper production, and the mining industry. This process involves the reaction of quicklime (calcium oxide, CaO) with water to form calcium hydroxide (slaked lime, Ca(OH)₂). The temperature at which lime slaking occurs plays a significant role in determining the efficiency and effectiveness of the reaction. This article will explore the effects of different temperatures on the lime-slaking process.
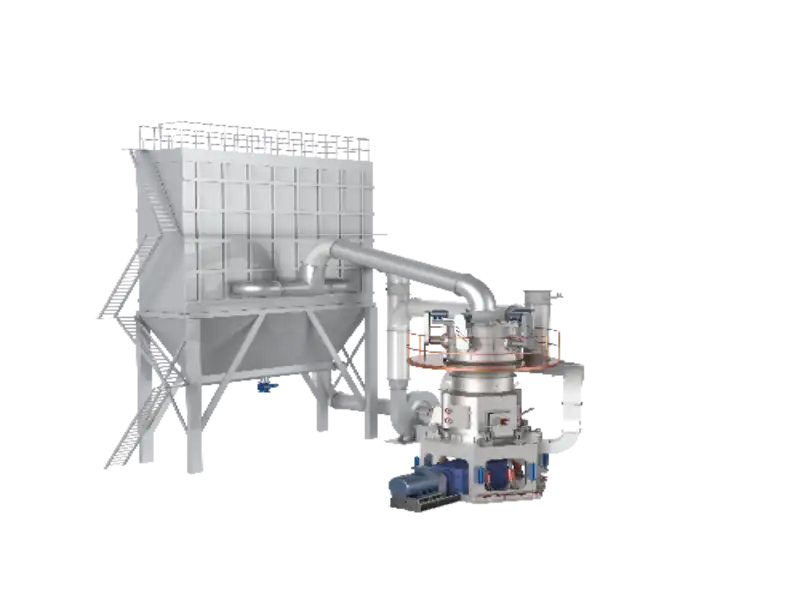
The main equipment for lime slaking process




Understanding the Lime Slaking Process
When quicklime is mixed with water, an exothermic reaction takes place:
[
\text{CaO} + \text{H}_2\text{O} \rightarrow \text{Ca(OH)}_2 + \text{Heat}
]
This reaction not only produces calcium hydroxide but also releases heat, which can influence the surrounding temperature and the kinetics of the reaction.
Effects of Temperature on Lime Slaking
- Low Temperatures (0°C – 25°C) At low temperatures, the rate of the lime slaking reaction is significantly decreased. The reaction may occur, but the formation of calcium hydroxide will be slower, leading to incomplete slaking. This can result in a residual amount of quicklime left unreacted, affecting the final product’s quality and purity. Moreover, lower temperatures can increase water viscosity, further slowing down the reaction kinetics.
- Moderate Temperatures (25°C – 60°C) As the temperature increases, the slaking process becomes more efficient. At moderate temperatures, the reaction rate increases due to enhanced molecular movement, leading to better contact between the quicklime and water. This results in a more complete conversion of quicklime to calcium hydroxide. The optimal conditions typically occur around this temperature range, providing a balance between reaction speed and calcium hydroxide yield.
- High Temperatures (60°C – 100°C) At high temperatures, the lime slaking process accelerates even further. The reaction can complete rapidly, and the heat generated can assist in maintaining a higher local temperature, thus promoting faster dissolution of quicklime. However, excessively high temperatures may lead to the formation of dry slaking or “burning” of the slaked lime, which can reduce the quality of calcium hydroxide produced. Moreover, operational safety becomes a concern as the risk of steam generation and pressure build-up increases.
- Very High Temperatures (above 100°C) At temperatures exceeding 100°C, the process can become problematic. The high heat may lead to rapid evaporation of water, making it difficult to maintain the necessary conditions for effective slaking. The risk of forming unreacted quicklime increases, along with potential hazards due to the pressure from steam. In most industrial applications, such conditions are avoided to ensure a controlled and safe slaking process.
Conclusion
Temperature plays a crucial role in the lime slaking process, influencing both the efficiency of the reaction and the quality of the final product. Maintaining optimal temperatures during slaking is essential for achieving the desired outcomes in various industrial applications. Understanding how temperature affects the slaking process can help industries optimize their operations, reduce waste, and improve product quality. Engineers and operators need to monitor and control the temperature to ensure safe and effective lime slaking.